
A Comprehensive Supply Chain for Core Intermediates in Diabetes Treatment
End-to-end supply chain for anti-diabetic intermediates. Covering DPP-4, SGLT-2 inhibitors & GPR40 agonists. Stable, compliant, scalable.
Table of Contents
In the fierce global battle for anti-infective drugs, bacterial resistance is increasing at an alarming rate, posing a major threat to human health. In this battle against drug-resistant bacteria, the β-lactamase inhibitor avibactam and its combination preparations have emerged as key weapons against drug-resistant Gram-negative infections.
Ceftazidime avibactam (CAZ-AVI) is a star drug in the anti-infective field. Approved by the U.S. Food and Drug Administration (FDA) in February 2015, it was successfully marketed in the United States for the treatment of Gram-negative infections, with particular efficacy against infections caused by difficult-to-treat-resistant (DTR) Gram-negative bacteria.
In May 2019, with approval from the National Medical Products Administration, it officially entered the Chinese market, providing a new treatment option for Chinese patients.
On May 8, 2025, Pfizer received another boost of good news for its injectable ceftazidime avibactam sodium, receiving approval from the National Medical Products Administration (NMPA).
Its scope of application has been expanded to include the treatment of complicated intra-abdominal infections (cIAI), hospital-acquired pneumonia, and ventilator-associated pneumonia (HAP/VAP) caused by Gram-negative bacteria in children of all ages, including newborns, as well as infections with limited treatment options, thus safeguarding the health of pediatric patients.
In April 2024, aztreonam-avibactam received approval in the EU for the treatment of serious infections caused by multidrug-resistant Gram-negative bacteria, including complicated intra-abdominal infections (cIAI), hospital-acquired pneumonia (including VAP), and complicated urinary tract infections (cUTI), bringing new hope to European patients.
In 2025, this innovative drug also achieved significant progress in China. On June 30th, the latest information from the official website of the National Medical Products Administration (NMPA) revealed that Pfizer’s aztreonam avibactam sodium for injection has been officially approved in China for the treatment of complicated intra-abdominal infections (cIAI) and hospital-acquired pneumonia (HAP), including ventilator-associated pneumonia (VAP), caused by Gram-negative bacteria in adults with limited or no alternative treatment options. This provides a more effective treatment option for adult patients in China.
Two months later, on August 13th, Pfizer’s new antibacterial drug, Sifuno® (aztreonam avibactam sodium for injection), was prescribed for the first time nationwide at several hospitals, including Gaobo Shanghai Zhaxin Hospital of Integrated Traditional Chinese and Western Medicine and Shanghai Liquan Hospital. This marked the drug’s official entry into clinical use, bringing tangible benefits to patients.
Facing the increasingly severe bacterial resistance situation and growing market demand, Shandong Tianming Pharmaceutical, leveraging its extensive technical expertise and strong R&D capabilities, has deeply engaged in the R&D and production of pharmaceutical intermediates, achieving significant breakthroughs in the production of key intermediates in the avibactam series.
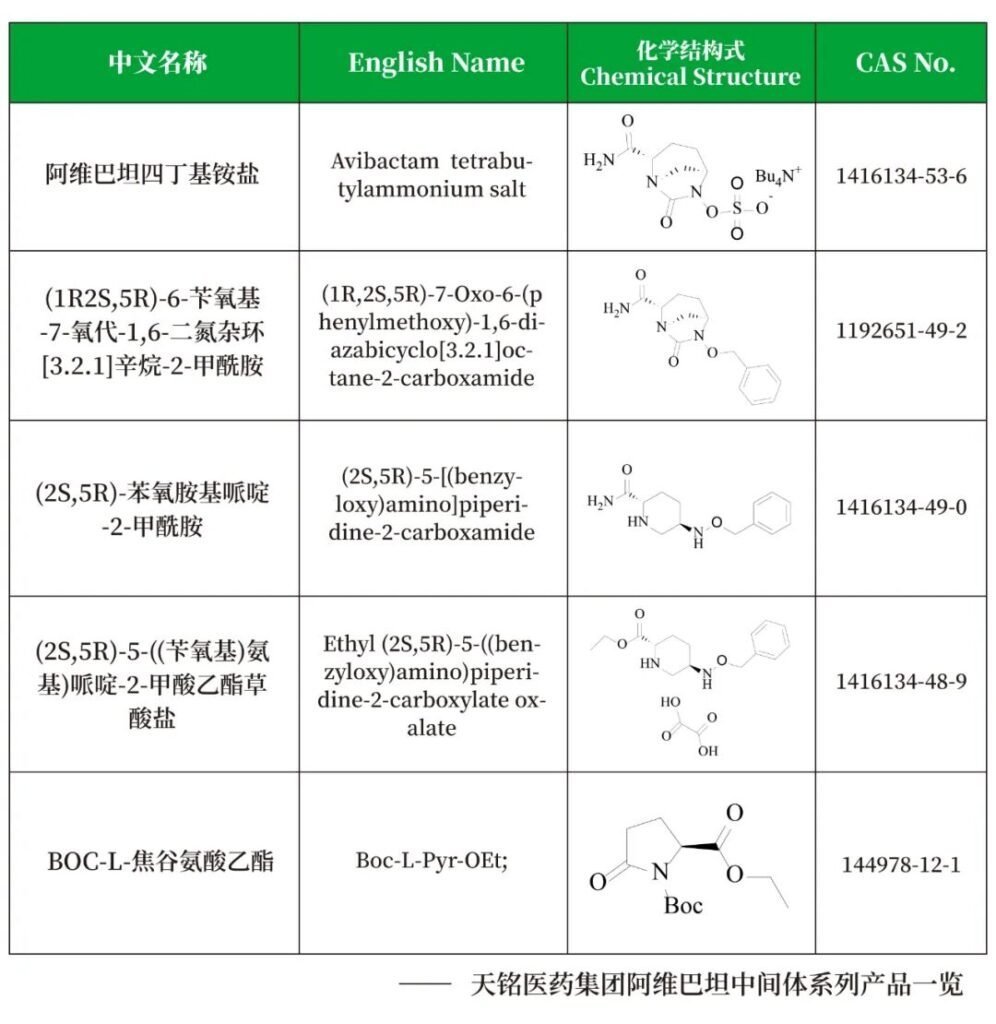
The company has successfully achieved large-scale commercial production of key intermediates for avibactam. Through continuous optimization and innovation, we have made significant technological progress in the synthesis of key intermediates such as (2S,5R)-5-[(phenylmethoxy)amino]-2-piperidinecarboxylic acid ethyl ester oxalate.
Our advanced production equipment and rigorous quality control system ensure that every batch of our products meets high international standards, providing a stable and reliable source of raw materials for downstream customers.
If you are interested in the production technology, application development, or other aspects of avibactam intermediates, or have any related collaboration needs, please feel free to contact us. We will provide detailed product information and technical support, and we can also request samples so you can experience the superior quality of our products firsthand.
sunqian0123@gmail.com

End-to-end supply chain for anti-diabetic intermediates. Covering DPP-4, SGLT-2 inhibitors & GPR40 agonists. Stable, compliant, scalable.
Drawing from on-the-ground experience in China’s top pharma clusters, this guide cuts through the jargon to reveal when to partner for complex innovation and when to buy from the workhorses of mature production.

This case study, based on publicly available academic literature and patents, focuses on the application of synthetic route optimization in improving the yield and purity of Active Pharmaceutical Ingredients (APIs).

Leading provider of high-quality APIs and intermediates. Contact us for innovative solutions and expert support.